Rechercher
Accueil > La Recherche > Axes & Equipes > Bio Nano Imagerie > Equipe : Biophotonique > Thème : Biophotonique
Photonics for bioimaging
publié le , mis à jour le
We develop and use spectroscopic and microscopic techniques to address the following collaborative subjects :
1. The action of anti-tumor drugs on cancer cells and on the organization of microtubules that are the target of chemo-therapy (CRLC Val d’Aurelle - INSERM U 896, UM1)
2. Early detection of oral cavity cancers based on the autofluorescence of malignant tissue (EA 4203, UM1)
3. Induction of neurogenesis in the treatment of peripheral sensory neuropathy (Institute of Neurosciences of Montpellier, INSERM U 583, UM2)
4. Dynamics of assembly of casein micelles (IATE - UMR 1208 INRA, CIRAD SUPAGRO)
In the frame of our collaborations with the “Centre de Recherche de Lutte Contre le Cancer, Val d’Aurelle” our objective is understanding tumour resistance mechanisms of cancerous cells to anti-tumoral drugs via direct observation of the cell morphology and its link with the microtubule behaviour and their dynamics regulation in vitro and in vivo. We employ combined label-free microscopic techniques to address understanding how the cytoskeleton and particularly the microtubules are influenced by anti-tumoral drugs. The morphology of living cells is studied by atomic force microscopy (AFM) in liquid phase. In the figure below one can note significant morphological changes due to the effect of an « onco-protective » treatment on cultured human breast cancer cells.
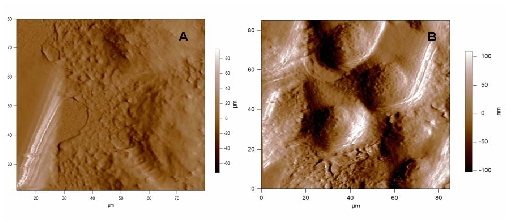
In addition to high-resolution imaging, AFM is designed to perform indentation measurements on biological or biomimetic nano-materials that are relatively soft and allow high quality dynamic spectroscopy measurements of forces too.1 Thus information can be obtained on the nature of the adhesion forces, but also on the mechanical properties of cells and other soft materials. By recording force-distance curves either at single, well-defined locations, or at multiple locations to yield a so-called ‘force volume image’. In doing so, spatially –resolved maps of properties (elasticity and adhesion) and molecular recognition interactions can be produced. The method presents the advantage of being a local probe technique to determine the local stiffness of a cell, by measuring its Young modulus. Our recent results in dynamic force spectroscopy AFM measurements suggest that cell elasticity is modified for cancerous cells.
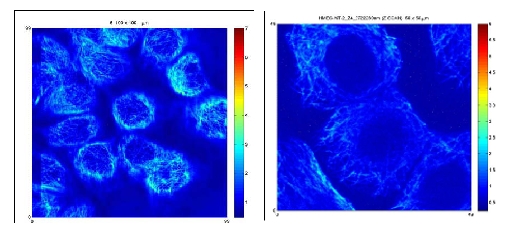
Due to the good penetration depth of IR light, multi-photon fluorescence microscopy has become a powerful tool in deep tissue imaging providing also in-depth images of cells. In addition to the fluorescence signals generated by the multi-photon excitation process, second harmonic generation (of microtubules too) provide useful and complementary information on the structure and optical properties of a specimen. Our results demonstrate the inherent capacity of the multiphoton microscopy to visualise microtubule bundles within the cells (figure below).
Through our collaboration with the “Peripheral nerve injury and regeneration” group of the Institute for Neuroscience of Montpellier (INM), INSERM U583 we study the regenerative growth of injured sensory neurons. The subject addresses the frequent post-traumatic neuropathies, which are often chronic and mostly resistant to current treatments. Following nerve injury, neurons have to adapt to a new environment in order to successfully promote their axonal elongation. The physical approach we adapted for these studies aim to monitor nanomechanics and electrical activity of neuronal live growth cones under various external conditions inducing a regenerative mode of growth. Multiphoton microscopy (MPM) is used for imaging cultured neurons, based partly on the response in the second harmonic generation (SHG) of microtubules localized in the axons. Moreover, the SHG signal proved to be essential for rapid optical recording of the membrane potential at higher resolutions compared to conventional measuring systems with electrodes. Consequently, the real-time monitoring of SHG of the neuronal cells can provide us unique information about their structures and the membrane potential at the same time. Neurons’ nanomechanics is addressed via cell-elasticity measurements with atomic force microscopy (AFM) providing also high resolution images on the morphology of the growth cones.3 Here are some recent results issued from our laboratory on live sensory neurons :
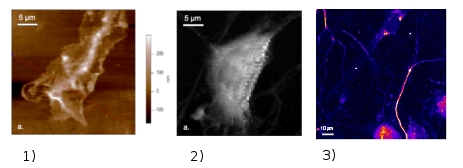
Use of functionalized probes in force mode AFM provides identification (mapping) of the receptors within the neurons cell membranes. These physical strategies will allow establishing a correlation between the optical response and the structure/function of neurons under different physical / chemical external conditions and in the presence of various factors inducing the growth of neurons. This should eventually lead to develop new rational approaches in the treatment of peripheral sensory neuropathy for which clinicians are missing often effective treatment.
Our studies at molecular and cellular level will be conveniently complement the magnetic resonance imaging (MRI) of neurons at the level of tissues and organs performed in the laboratory of C. Goze-bac (L2C).
Collaborations : C. Larroque (CRLC Val d’Aurelle - INSERM U 896, UM1), F.J.G. Cuisinier (EA 4203, UM1), F. Scamps, J. Valmier (Institute of Neurosciences of Montpellier, INSERM U 583, UM2), S. Marchesseau (IATE - UMR 1208 INRA, CIRAD SUPAGRO), C. Goze-bac (L2C), M. Zanca (CHU Montpellier)








